Aqueous Sodium Chloride Reacts With Aqueous Lead Ii Nitrate
LeadII nitrate reacts with sodium chloride in aqueous solution to form a precipitate. PbNa2 If the molality of a solution of NaCl aq is 170 m what is the percent by mass of NaCl in the solution.

Solved 13 When Sodium Chloride And Lead Ii Nitrate React Chegg Com
X does not react with aqueous sodium chloride.

. To balance Pb NO32 NaCl PbCl2 NaNO3 youll need to be sure to count all of atoms on each side of the chemical equation. Lead II nitrate and sodium iodide react to form sodium nitrate and lead II iodide. Sodium chloride lead II nitrate lead II chloride sodium nitrate.
Up to 256 cash back Aqueous potassium chloride will react with which one of the following aqueous solutions to produce a precipitate. The precipitate lead chloride is insoluble in cold water but it is soluble in hot water. Balance the equation and classify it.
Pb NO32 aq2NaI aqPbI2 s2NaNO3 aq You have already submitted this answer. Click to see full answer. Enter a balanced equation for the reaction between aqueous lead II II nitrate and aqueous sodium chloride to form solid lead II II chloride and aqueous sodium nitrate.
If the precipitate contains lead write the balanced chemical equation forthis reaction. MgNO32aq CaCl2aq --. CPb2 aq2Cl- aqPbCl s.
Select the precipitate that forms. When sodium chloride solution is added to lead nitrate solution then it results in the formation of a precipitate of lead chloride and sodium nitrate. Solid lead IV oxide decomposes into solid lead II oxide and oxygen.
Thus silver nitrate is soluble but silver chloride precipitates from solution as a curdy white solid. I used the textbook 1. Likewise does a reaction occur when aqueous solutions of Chromium II.
Terms in this set 28 Balance the equation and classify it. What occur when sodium chloride is heated. As all possible products are aqueous there is no reaction between aqueous magnesium nitrate and aqueous calcium chloride.
Express your answer as a chemical equation. When NaCl is heated it will get scorching then at 801C it melts and at 1413C it boils and when its molten It may well conduct Electrical energy. Aqueous sodium chloride reacts with aquaeous lead II nitrate to yield a lead II chloride precipitate and aqueous sodium nitrate.
No reaction Calcium nitrate and sodium. Calculate the mass of lead II sulfate that should form when 125 L of 00500 M PbNO32 and 200 L of 00250 M. Be sure to include the states of each of the reactants and products.
Identify all of the phases in your answer. Are my answers below correct. Aqueous lead II nitrate PbNO32 undergoes a double displacement reaction with aqueous sodium chlorideNaCl in which a precipitate forms.
When aqueous solutions of sodium sulfate and lead II nitrate are mixed lead sulfate precipitates out of soultion. Aqueous solutions of leadII nitrate and sodium chloride react to form solid leadII chloride and aqueous sodium nitrate according to the reaction below. XO reacts with magnesium to form X.
Select the precipitate that forms when aqueous lead II nitrate reacts with aqueous sodium chloride. X reacts with aqueous silver nitrate. Aqueous barium nitrate reacts with sulfuric acid H 2 SO 4.
PbNO 3 2 aq 2 NaCl aq 2 NaNO 3 aq PbCl 2 s What is the molarity of the sodium chloride solution if 880 mL of it will produce 695 g of the leadII chloride. NaCl PbNO32 PbCl2 NaNO3. Silver nitrate and sodium chloride will react in aqueous solution to produce silver chloride an insoluble solid that precipitates out of the solution and aqueous sodium nitrate.
See the Expert Answer. A Use the information to help arrange the following metals in order of reactivity. When sodium chloride solution is added to lead nitrate solution then it results in the formation of a precipitate of lead chloride and sodium nitrate.
The reaction equation for this chemical reaction is as follows. How to balance sodium chloride and lead II nitrate. When sodium chloride and leadII nitrate react in an aqueous solution which of the following terms will be present in the balanced molecular equation.
Lead magnesium silver sodium and X. The reaction equation for this chemical reaction is as follows. Aqueous sodium chloride reacts with aqueous lead II nitrate to yield a lead II chloride precipitate and aqueous sodium nitrate sodium chloride lead II nitrate lead II chloride sodium nitrate NaCl PbNO 3 2 PbCl 2 NaNO 3 2 NaCl aq PbNO 3 2 aq PbCl 2 ppt 2 NaNO 3 aq 2.
AgNO_3aq NaClaq rarr NaNO_3aq AgClsdarr This reaction is commonly used to illustrate basic solubility rules and solubility equilibria. The precipitate lead chloride is insoluble in cold water but it is soluble in hot water. When hydrochloric acid resolution reacts with lead lI nitrate resolution lead ll chloride precipitates and a resolution of nitric acid is produced.
Add your answer and earn points. 2 NaCl Pb NO32 PbCl2 2 NaNO3. Aqueous solutions of leadII nitrate and sodium chloride react to form solid leadII chloride and aqueous sodium nitrate according to the reaction below.
Calcium nitrate sodium bromide lead II nitrate barium nitrate sodium chloride Write the balanced molecular equation for the reaction that occurs IN PROBLEM 1. Asked Jun 19 2017 in Chemistry by rakuman78 general-chemistry. The balanced chemical equation is PbNO32 2NaI 2NaNO3 PbI2.
Aqueous sodium chloride reacts with aqueous lead II nitrate to yield a lead II chloride precipitate and aqueous sodium nitrate. PbNO 3 2 aq 2 NaCl aq 2 NaNO 3 aq PbCl 2 s What is the molarity of the sodium chloride solution if 750 mL of it will produce 601 g of the leadII chloride. What is the balanced chemical equation for the reaction of aqueous calcium chloride with aqueous sodium carbonate to produce calcium carbonate precipitate in an aqueous sodium chloride solution.
Balanced equation for the reaction of lead ii nitrate and sodium chloride. What is the net ionic equation for this reaction. And all halides are soluble EXCEPT for AgX PbX_2 and Hg_2X_2.
All nitrates are soluble hence silver nitrate is soluble. The mixing of which pair of reactants will result in a precipitation. X reacts with aqueous leadII nitrate.

Solved Aqueous Sodium Oxalate And Aqueous Lead Ii Nitrate React To Produce Solid Lead Ii Oxalate And Aqueous Sodium Nitrate
How To Write An Ionic Equation For Sodium Sulfate And Lead Nitrate Quora
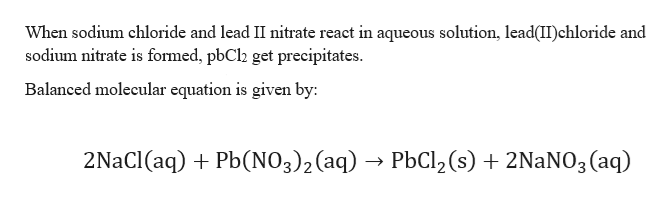
Answered When Sodium Chloride And Lead Ii Bartleby
Solved 15 Question 20 5 Points Lead Ii Nitrate Reacts Chegg Com

How To Write The Net Ionic Equation For Na2s Pb No3 2 Nano3 Pbs Youtube

Lead Ii Nitrate Reaction With Potassium Iodide Pb No3 2 Ki Youtube
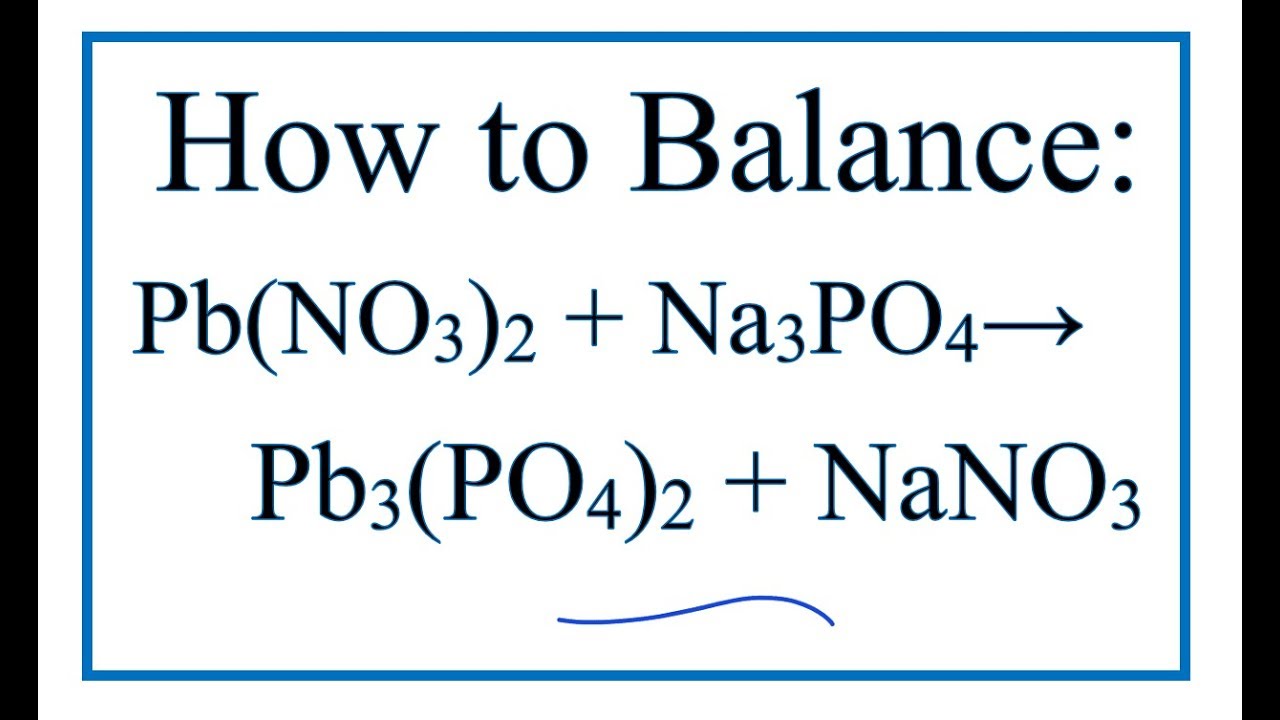
How To Balance Pb No3 2 Na3po4 Pb3 Po4 2 Nano3 Lead Ii Nitrate Sodium Phosphate Youtube

How To Write The Net Ionic Equation For Pb No3 2 Na3po4 Pb3 Po4 2 Nano3 Youtube

How To Balance Pb No3 2 Nabr Pbbr2 Nano3 Lead Ii Nitrate Sodium Bromide Youtube

How To Write The Net Ionic Equation For Pb No3 2 Nacl Youtube

Solved When Sodium Chloride And Lead Ii Nitrate React In Chegg Com

Solved Question 33 1 Point When Sodium Chloride And Chegg Com

A Solution Of Sodium Chloride Is Mixed With A Solution Of Lead Ii Nitrate A Precipitate Of Homeworklib

How To Balance Pb No3 2 Nacl Pbcl2 Nano3 Youtube

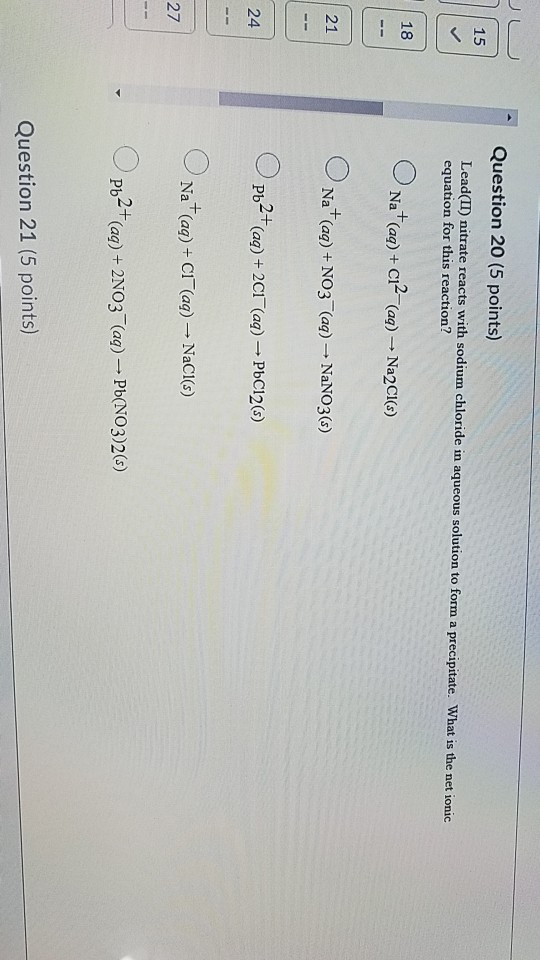
Solved Lead Ii Nitrate Reacts With Sodium Chloride In Aqueous Solution T0 Form Precipitate What Is The Net Ionic Equation For This Reaction Na Aq No Aq Nano S Pb T Aq 2ci Aq Pbclz S Na Aq
Solved Aqueous Lead Ii Nitrate Pb No3 2 Undergoes A Double Displacement Course Hero

How To Write The Net Ionic Equation For Pb No3 2 Na2cro4 Pbcro4 Nano3 Youtube
Comments
Post a Comment